Renal Function Outcomes with Everolimus Plus Reduced-Exposure Tacrolimus in de Novo Living Donor Liver Transplantation: 24-month results from the H2307 study
Kyung Suk Suh1, Long-Bin Jeng2, Arvinder Singh Soin3, Wei-Chen Lee4, Sung Gyu Lee5, David Grant6, Tomoharu Yoshizumi7, Mahmoud El Meteini8, Matthias Meier9, Jossy Kochuparampil9, Carole Sips9, Shuhei Kaneko10, Gary Levy6.
1Seoul National University College of Medicine, Seoul, Korea; 2China Medical University Hospital, Taichung, Taiwan; 3Medanta, Medicity Hospital, Gurgaon, India; 4Chang Gung Memorial Hospital, Taoyuan, Taiwan; 5Asan Medical Center, Seoul, Korea; 6University of Toronto, Toronto, ON, Canada; 7Kyushu University Hospital, Fukuoka, Japan; 8Ain-Shams University, Cairo, Egypt; 9Novartis Pharma AG, Basel, Switzerland; 10Novartis Pharma KK, Tokyo, Japan
CRAD0001H2307.
Introduction: Early initiation of everolimus (EVR) with reduced-exposure tacrolimus (rTAC) has been shown to maintain an efficacy and safety profile comparable to that of a standard-exposure TAC (sTAC) regimen and preserves renal function in living-donor liver transplant recipients (LDLTRs) up to 12 months post-LT. Here, we present 24-month (M) renal function outcomes from the H2307 study involving LDLTRs receiving EVR+rTAC or sTAC regimens.
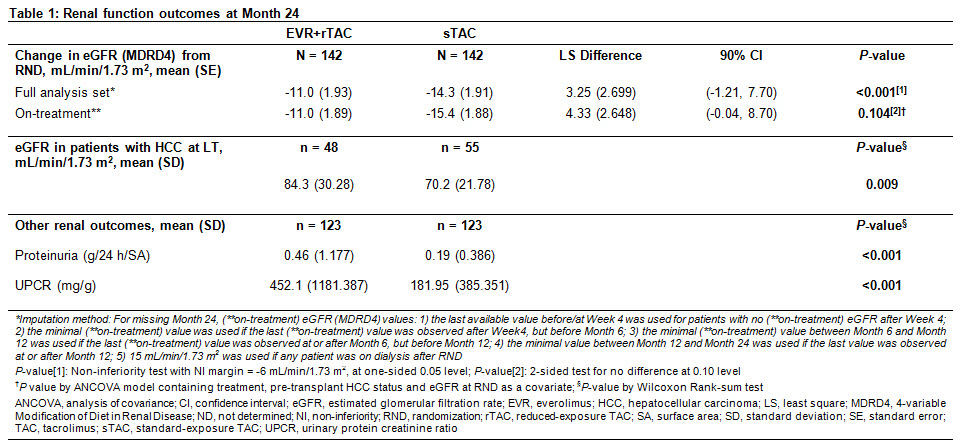
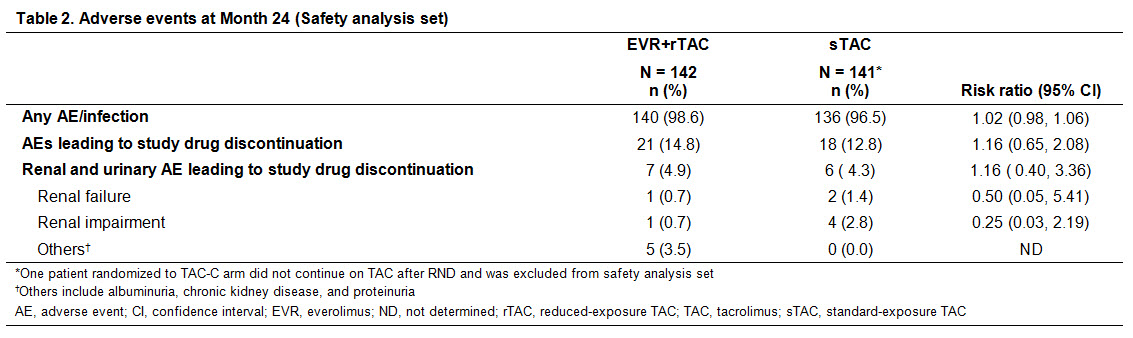
Methods: H2307 (NCT01888432) was a 24M, multicentre, open-label trial in which 284 adult de novo LDLTRs were randomized (1:1) on Day 30±5 post-LT to receive EVR+rTAC (EVR trough level [C0]: 3-8 ng/mL; TAC C0: 3-5 ng/mL) or sTAC (TAC C0: randomization (RND)-M4: 8-12 ng/mL; after M4: 6-10 ng/mL) regimen. Efficacy assessment at M24 was incidence of composite efficacy failure of treated biopsy-proven acute rejection, graft loss, or death in EVR+rTAC and sTAC arms. Renal assessments at M24 included estimated glomerular filtration (eGFR [MDRD4]) in overall study population and in patients with hepatocellular carcinoma (HCC). Other assessments included evaluation of proteinuria and renal adverse events (AEs) up to M24.
Results: Overall results are presented in Table 1. Of 284 randomized patients, 88% in both arms completed the 24M study. At M24, 78% of patients in EVR+rTAC arm were within EVR C0 range although mean TAC C0 was below target range in sTAC arm. EVR+rTAC was non-inferior to sTAC for the primary endpoint of composite efficacy failure at M24 (9.0% vs 8.0%; P<0.001 by one-sided test for non-inferiority). In the overall population, eGFR was comparable between EVR+rTAC and sTAC (78.4 and 75.3 mL/min/1.73 m2) arms, with between-arm least square mean change from RND to M24 not significantly different in the full analysis and on-treatment populations. Among patients with HCC at LT, eGFR was significantly higher in the EVR+rTAC arm (P = 0.009). In overall population, proportion of patients with M24 eGFR ≥60 mL/min/1.73 m2 was higher in the EVR+rTAC (76.4%) vs sTAC (66.9%) arm. Although mean proteinuria and urinary protein creatinine ratio (UPCR) were significantly higher in EVR+rTAC vs sTAC arm (P<0.001), most patients in both arms remained in the low-to-mild categories for proteinuria (>80% with <0.5 g/24 h/SA) and UPCR (>70% with ≥30-<300 mg/g) at M24. Incidence of AEs and AEs leading to study drug discontinuation was comparable between arms; however, discontinuations due to renal AEs such as renal failure and renal impairment were numerically lower in EVR+rTAC vs sTAC arm (Table 2).
Conclusions: At M24, early EVR initiation with rTAC in LDLTRs resulted in comparable renal function outcomes as sTAC regimen. Significantly better renal function was observed in patients with HCC on EVR+rTAC, which warrants additional analysis.