Repeated Freezing and Thawing Does Not Affect HLA Antibody Titer
Myoung Hee Park1, Jiye Kwak1, Hye Young Sung1, Sohyun Kim1, Boknyun Han1.
1Laboratory, Korea Organ Donation Agency, Seoul, Korea
Introduction: For deceased donor organ transplantations, we are not currently using virtual crossmatches (XMs) and preliminary negative T cell XMs are mandatory for kidney and pancreas allocation in Korea. Korean Network for Organ Sharing (KONOS) distributes transplant candidates’ sera to 21 OPO laboratories and KONOS preliminary XMs are performed in these laboratories using frozen stored sera. We are performing more than 40% of nationwide KONOS XMs in the Korea Organ Donation Agency (KODA) laboratory, and we had difficulties in keeping the frozen sera from more than 20,000 transplant candidates in small aliquots to avoid HLA antibody deterioration. We investigated the extent of change of antibody titer after repeated freezing and thawing.
Materials and Methods: Two different sera (serum A and B), which were tested positive for both class I and class II HLA antibodies were selected for the investigation. Sodium azide (0.1% in final concentration) was added to the sera as preservative, and freezing-thawing cycles were repeated for 20 times. A total of 7 serum samples (1, 3, 6, 9, 12, 15, and 20 times frozen-thawed; labeled #1, #3, #6, #9, #12, #15, and #20) were used for HLA antibody screening test (One Lambda, USA), and 3 samples (1, 9, and 20 times frozen-thawed) were used for HLA antibody single antigen assay (One Lambda, USA) and T cell flow cytometry XM (FCXM) test. To avoid prozone phenomenon, 1:1 (neat), 1:5, and 1:20 diluted sera were tested by screening assay and 1:20 sera for both serum A and B were used for the investigation. HLA antibody strength in mean fluorescence intensity (MFI) values and FCXM result in MFI ratios were compared. Test results of #20 sample compared to #1 sample were calculated as percentage values and variabilities of the test results among different samples of repeated freezing-thawing were calculated as coefficient of variation (CV) values.
Results and Discussion: The results are summarized in Table 1. There was little change in MFI values after 20 times of repeated freezing-thawing cycles: percentage of #20 vs # 1 sample as baseline (100%) was in the range of 99-110% for screening assay and 90-136% for single antigen assay. For serum A, change of MFI values of 5 class I and 5 class II antibodies of the #1, #9 and #20 samples are shown (Fig. 1 and Fig. 2). Variability of the test results among different samples of repeated freezing-thawing was low, and most of the CV values (7 out of 8) were <10% (2.2-6.9%). T cell FCXM results (3 XMs) also showed little change in MFI ratios (103-107%) and low CV values (4.2-6.7%). Unlike the notion that antibodies are deteriorated after repeated freezing-thawing, the strength of HLA antibodies was little affected after 20 times of freezing-thawing cycles. 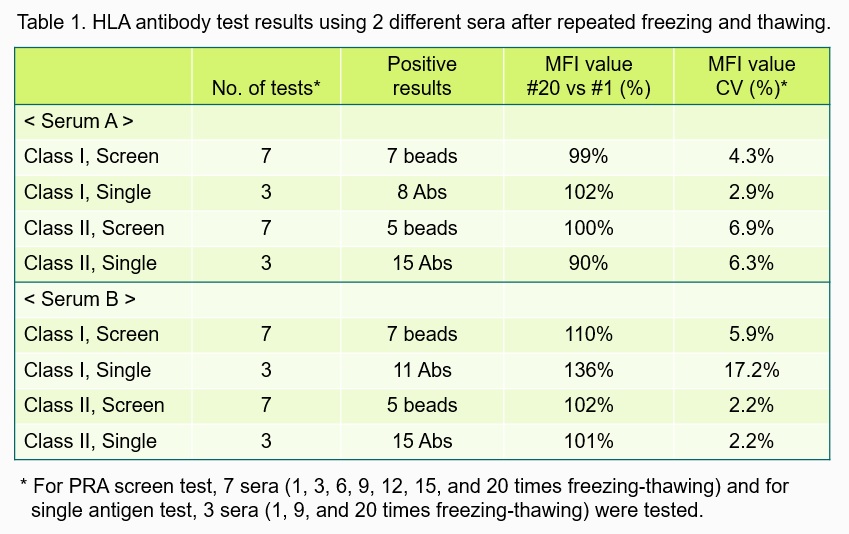
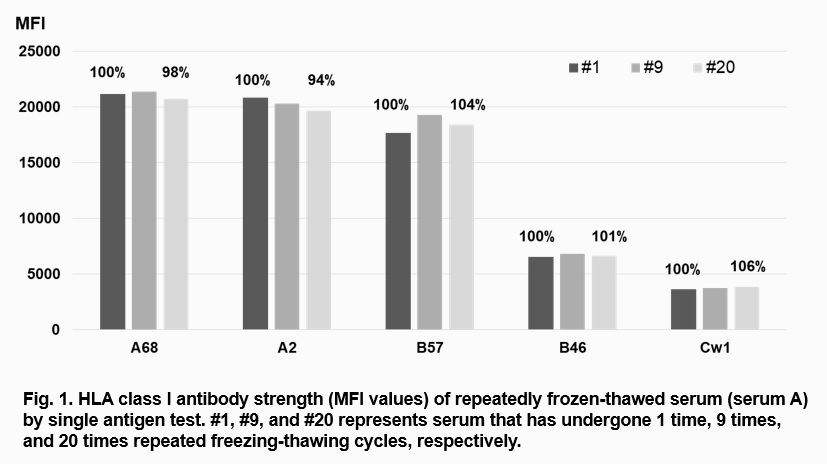
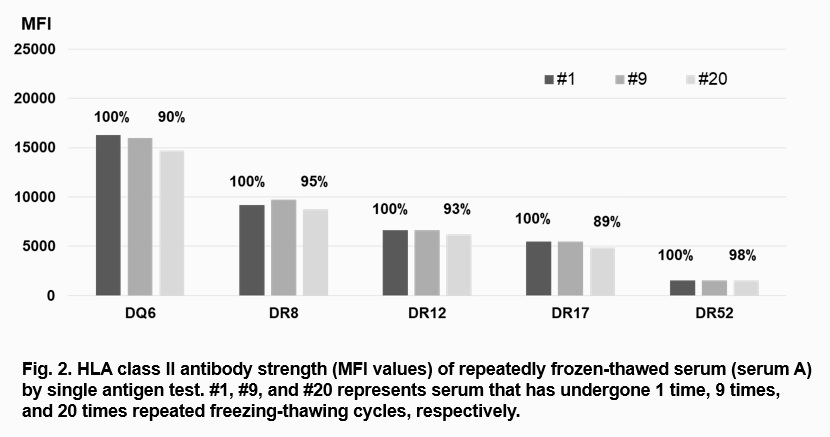
Conclusion: HLA antibody strength is little affected by up to 20 times of repeated freezing and thawing. For preliminary HLA XM tests for deceased donor organ transplantation, serum samples from transplant candidates can be stored frozen without aliquoting.
