Single Centre Experience with the use of Envarsus in Pediatric Kidney Transplant Population
Anna Vila-Santandreu1, Yolanda Calzada 1, Elena Codina 1.
1Pediatric Nephrology Department , Hospital Sant Joan de Deu , Esplugues De LL, Spain
Introduction: There is no published experience with the use of Meltdose Tacrolimus (M-TAC) in pediatric transplant population. Theoretically, this new drug should not only improve adherence to treatment, but it could also offer better pharmacokinetics than once-daily extended-release tacrolimus (ER-TAC) with less side effects.
Patients and Methods: This is a retrospective study of a single centre experience with the use of M-TAC in pediatric stable kidney transplant recipients who were selectively converted from other tacrolimus (TAC) formulae to M-TAC. Sixteen patients (44% boys, mean age at transplant 9.3∓5.6 years, mean time after transplant of 2.7 years) were switched from Advagraf(R) or Prograf(R) to M-TAC. The reasons for conversion were: improve TAC pharmacokinetics (56%), improve patient's adherence to treatment (25%) or reduce TAC adverse events (19%).
The immunossupression regime prior to conversion was TAC and prednisone based-triple therapy in 81% of them and double therapy in 19%.
Results: Mean pre-conversion TAC dose was 7.03∓4.3 mg/day. Mean post-conversion TAC dose was 5.03∓2.9 mg/day, with a mean Conversion Factor of 0.73∓0.08. Mean pre-conversion TAC through blood level was 7.52∓2.75 ng/ml (range 4.4 - 12.7) and the post-conversion mean level was 7.81∓2.78 ng/ml (range 3.2 - 15.4)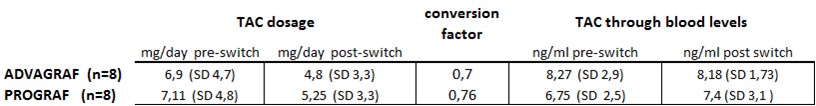
At a mean time of 15 months of follow-up after conversion 94% of the patients are still on M-TAC treatment. A new onset diabetes (NODAT) was diagnosed 3 months after conversion and it disappeared after the M-TAC withdrawal. No clinical acute rejection events have been diagnosed after the conversion in this population. No beneficial effect was observed in 3 patients who persist showing high variability on TAC through blood levels.
Conclusions: The switch from other TAC formulae to Meltdose TAC using the recommended conversion factor of 0.7 can be safely done also in children. The through blood levels obtained after conversion are quite predictable and stable. The switch can be done without increasing the risk of rejection episodes.
