
Development of Recommendations for a Core Outcome Set for Clinical Trials of Physical Rehabilitation in Adults Across the Continuum of Solid Organ Transplantation: a Canadian Delphi Consensus Study
Tathiana Santana-Shiguemoto1, Sunita Mathur1,2, Tania Janaudis-Ferreira2,3, Sabrina Figueiredo3.
1Dept of Physical Therapy, University of Toronto, Toronto, ON, Canada; 2Canadian National Transplant Research Program, University of Alberta, Edmonton, AB, Canada; 3School of Physical and Occupational Therapy, McGill University, Montreal, QC, Canada
Introduction: Clinical trials of physical rehabilitation (PR) in solid organ transplant (SOT) use varied outcome measures, which creates difficulties comparing and combining findings across studies. A core outcome set (COS), defined as “the minimum agreed set of outcomes that should be measured and reported in all clinical trials of a specific disease or trial population”, can address this issue. The purpose of this study was to develop consensus-based recommendations on standard COS for clinical trials of PR in adult SOT patients for three phases: pre-transplant, early post-transplant, late post-transplant.
Material and Methods: A Delphi procedure was used to reach consensus on the most important outcome domains for clinical trials of PR and SOT. The protocol was registered with COMET initiative (http://www.comet-initiative.org/). Panellists consisted of patients (SOT recipients); clinicians and researchers involved in the field of SOT rehabilitation in Canada. Panellists were provided with a list of 27 outcome domains based on a systematic review of exercise trials in SOT. Panellists were asked to rate the importance of outcome domains using a 9-point Likert scale ranging from “not important” to “very important-critical”. Three rounds of online questionnaires were performed to obtain consensus. In round 1, consensus for including an outcome was defined by 70% or more participants scoring “very important” and less than 15% scoring “not important”. In rounds 2 and 3, consensus was reached when 60% or more participants scored an outcome as “very important” and less than 15% scored it “not important”, within at least one subgroup of patients or clinicians/researchers.
Results and Discussion: Forty panellists were included in the study (25 clinicians and researchers and 15 patients); 33 completed all 3 rounds (response rates of 95% for rounds 1 and 2, and 92% for round 3). Five outcomes reached consensus in the pre-transplant phase; 10 in the early post-transplant phase and 8 in the late post-transplant phase (see Figure). Exercise capacity, mobility, quality of life, and frailty reached consensus across the 3 phases of transplant. 20% of the outcomes were related to body function and structure in the pre-transplant phase, 40% in the early- and 13% in the late- post-transplant phases. Differences were noted in the ratings between patients and clinicians/researchers, especially in the early post-transplant phase, where patients rated outcomes related to body function and structure (such as organ rejection and infections) as having greater importance than clinicians/researchers.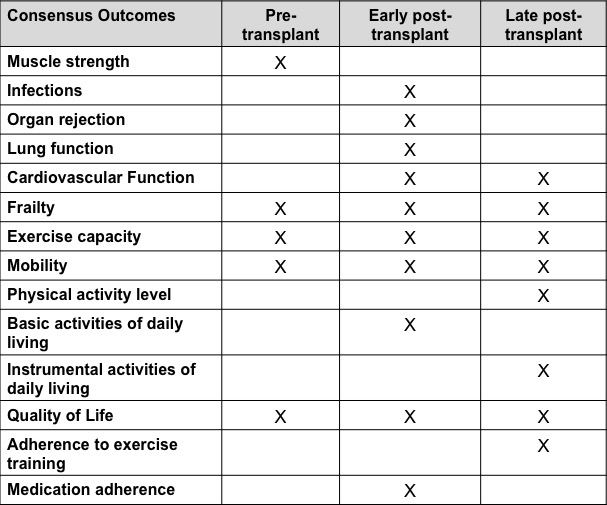
Conclusion: This study provides recommendations on COS domains that should be assessed in clinical trials of PR programs in adults across the continuum of SOT. Future steps include the selection of outcome measure instruments that match the domains identified in this study, and to expand the panel to an international group of experts.
