Prevention of Ischemic Injury in Colon and Small Intestine in an Ex Vivo Perfusion Animal Model: the Role of l-arginine and Nutraceutical Nanoparticles
Michele Finotti1,2, Taras Lysyy1, Maria J Barahona1, Renee M Maina1, Giorgio Caturegli1, Francesco D'Amico1,2, David Mulligan1, John P. Geibel1.
1Surgery, Yale, New Haven, CT, United States; 2Hepatobiliary Surgery, University of Padova, Padova, Italy
Introduction: The small intestine and colon are among the most sensitive organs to ischemic injury in the abdominal cavity, making them the least viable transplantable organs. It is imperative to investigate methods of preserving intestinal viability and mitigating ischemia-reperfusion injury (IRI). Using an ex-vivo intestinal perfusion machine we created a controlled ischemic injury and we hypothesized that perfusing the intestine with different solutions would reduce intestinal ischemic damage in rats.
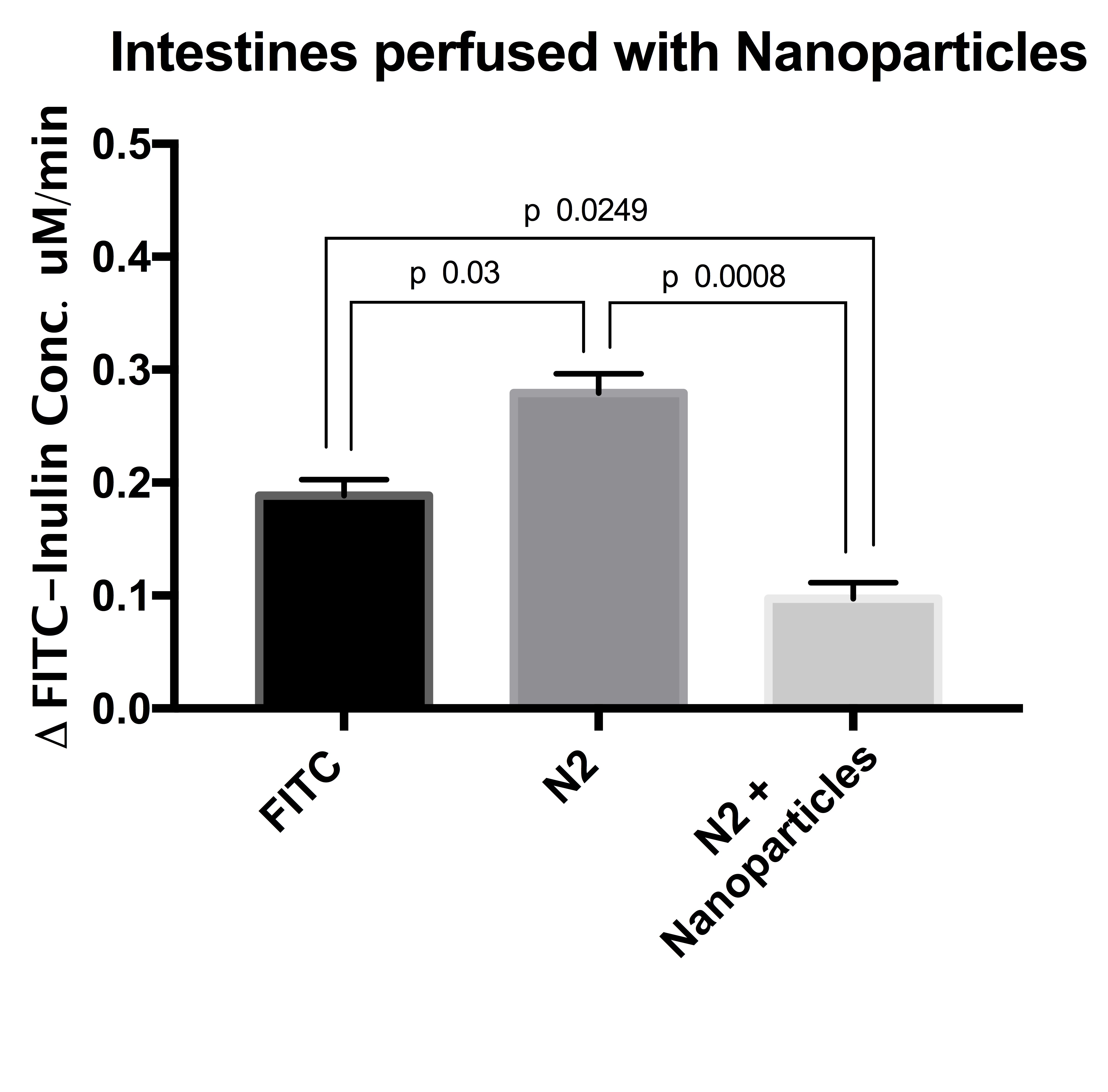
Materials and Methods: Small intestine (proximal, middle, and distal segments) and distal colon were isolated from male Sprague-Dawley rats. Each segment was connected to an ex-vivo intestinal perfusion machine that independently perfused the segments intraluminally and extraluminally. Controlled ischemic injury was induced by extraluminal perfusion with HEPES buffer saturated with 100% N2. The intraluminal side was perfused with two different conditions: 1) with and without L-Arginine, 2) with and without Ca2+ nanoparticles.
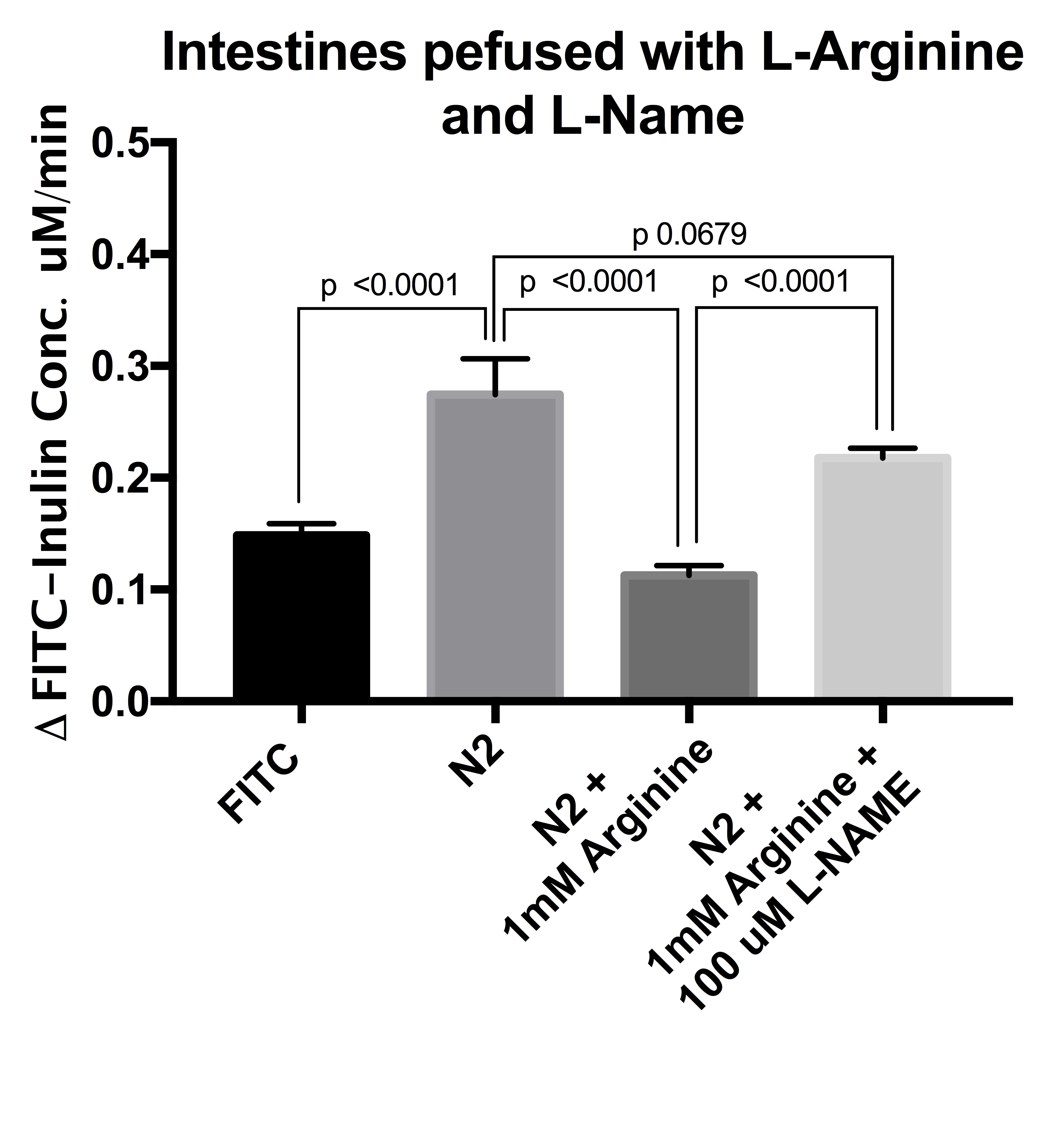
In a series of experiments, we used L-Arginine and L-NAME, a known antagonist of NO production.
In all experiments, FITC-inulin was used to assess the ischemic conditions of the intestinal grafts. The intestine and colon are non-permeable to inulin, thus fluorometry of samples served as an indicator of ischemic damage.
Results and Discussion: We conducted experiments in 62 intestine segments. Intestinal and colon segments exposed to 100% N2 showed significant drop in fluorescent signal, indicating ischemic damage (p<0.0001). A 100% N2 environment caused ischemic/inflammatory damage to the intestinal wall, resulting in cell swelling, loss of homeostatic parietal fluid control, and enhanced intraluminal secretion.
In the segments perfused with L-Arginine or calcium nanoparticles, ischemia was prevented and the fluorescence signal was similar to control perfused segments not exposed to N2 (p<0.0001 and p=0.0008 respectively, Fig 1&2). The presence of L-NAME reversed the protective effect of L-Arginine, and the degree of ischemic damage was not statistically significant to the segments exposed to 100% N2 and perfused without arginine.
Conclusion: The addition of L-Arginine and nanoparticles to the perfusate prevents ischemic injury in distal colon and all segments of the small intestine in an ex vivo animal perfusion model. L-NAME blocks this protective anti-ischemic effect of L-Arginine. In intestinal ischemic injury, several death signals activate the apoptotic and inflammatory pathways and result in the production of reactive oxygen species. We show that L-Arginine successfully prevents this via the NO production pathway. Nanoparticles targeting the CaSR also mitigate ischemic damage in the intestine. These results suggest that the use of L-Arginine and nanoparticles may be a potential method of reducing ischemic and inflammatory injury during intestinal harvesting/transport perfusion.